A matter of mobility: multidisciplinary paper suggests new strategy for drug discovery
A joint industry/academia study of a cancer target protein reveals unusual relation between binding site flexibility and drug-target lifetime. The results, published in “Nature Communications”, suggest a new strategy for drug discovery. The research was done in the framework of the Kinetics for Drug Discovery K4DD consortium, supported by the Innovative Medicines Initiative.
Most drugs exert their therapeutic effect by binding to a target protein molecule, thereby interfering with the normal function of the protein. Traditionally, it has been considered that the more tightly a drug binds its target protein, i.e. the greater its binding affinity is, the more effective it will be. However, drugs must function in the constantly changing environment of living organisms. It is therefore increasingly recognised that not only binding affinity and thermodynamics, but also drug-target residence times and kinetics must be optimized during the process of drug discovery.
Kinetics put to the test: Studying a cancer target
A multidisciplinary team of scientists from K4DD partners Merck KGaA (Darmstadt), Heidelberg Institute for Theoretical Studies (HITS), and the Instituto de Biologia Experimental e Tecnológica (iBET) (Lisbon), applied state-of-the-art experimental and computational approaches to investigate the determinants of target residence times for a set of inhibitors of a widely studied cancer target, heat shock protein 90 (HSP90). HSP90 inhibitors can disrupt the cell cycle and potentially stop tumour growth. The team recently published some of their results in Nature Communications.
Surprising results: greater binding site mobility leads to longer residence times
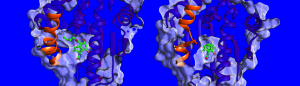
“At the moment, there is little known about the factors influencing drug-target residence times so we decided to measure the binding thermodynamics and kinetics, solve the structures of HSP90-inhibitor complexes and simulate their dynamics.” says Dr. Marta Amaral, one of the corresponding authors. The structures determined by x-ray crystallography show that the binding pocket of HSP90 is lined by a region that can take the form of a helix or a loop when bound to different inhibitors (see image below). The researchers found that compounds binding with a helix present bind for a longer time. “We were really surprised”, says Prof. Rebecca Wade (HITS), “when we found out that an important contributor to the long residence times was the greater mobility of the helical region of the binding pocket when the inhibitor bound.” This unusual binding mechanism opens a new avenue for drug design: Scientists can consider less rigid protein targets and identify molecules that stabilize more mobile forms of the protein upon binding – somewhat like a ski boot with an adaptable inner liner that continually adjusts to the foot. The findings of this study suggest a new way to find more effective drug candidates with optimal kinetic and thermodynamic properties.
Publication:
Protein conformational flexibility modulates kinetics and thermodynamics of drug binding
Amaral M, Kokh D, Wegener A, Bomke J, Buchstaller HP, Eggenweiler HM, Matias P, Wade RC, Frech M.
Nature Communications 8, Article number: 2276 (2017) doi:10.1038/s41467-017-02258-w
About K4DD
The Kinetics for Drug Discovery (K4DD) consortium of 20 partners was initiated in 2012 as a joint industry and academia endeavor to enable the adoption of drug-target binding kinetics analysis in the drug discovery decision-making process, and thereby contribute to the development of a new generation of improved medicinal products. K4DD has been supported by the Innovative Medicines Initiative Joint Undertaking (IMI JU) under grant agreement n° [115366], resources of which are composed of a financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution.
About Merck
Merck is a leading science and technology company in healthcare, life science and performance materials. Around 50,000 employees work to further develop technologies that improve and enhance life – from biopharmaceutical therapies to treat cancer or multiple sclerosis, cutting-edge systems for scientific research and production, to liquid crystals for smartphones and LCD televisions. In 2015, Merck generated sales of € 12.85 billion in 66 countries. Founded in 1668, Merck is the world’s oldest pharmaceutical and chemical company. The founding family remains the majority owner of the publicly listed corporate group. Merck, Darmstadt, Germany holds the global rights to the Merck name and brand. The only exceptions are the United States and Canada, where the company operates as EMD Serono, MilliporeSigma and EMD Performance Materials.
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.