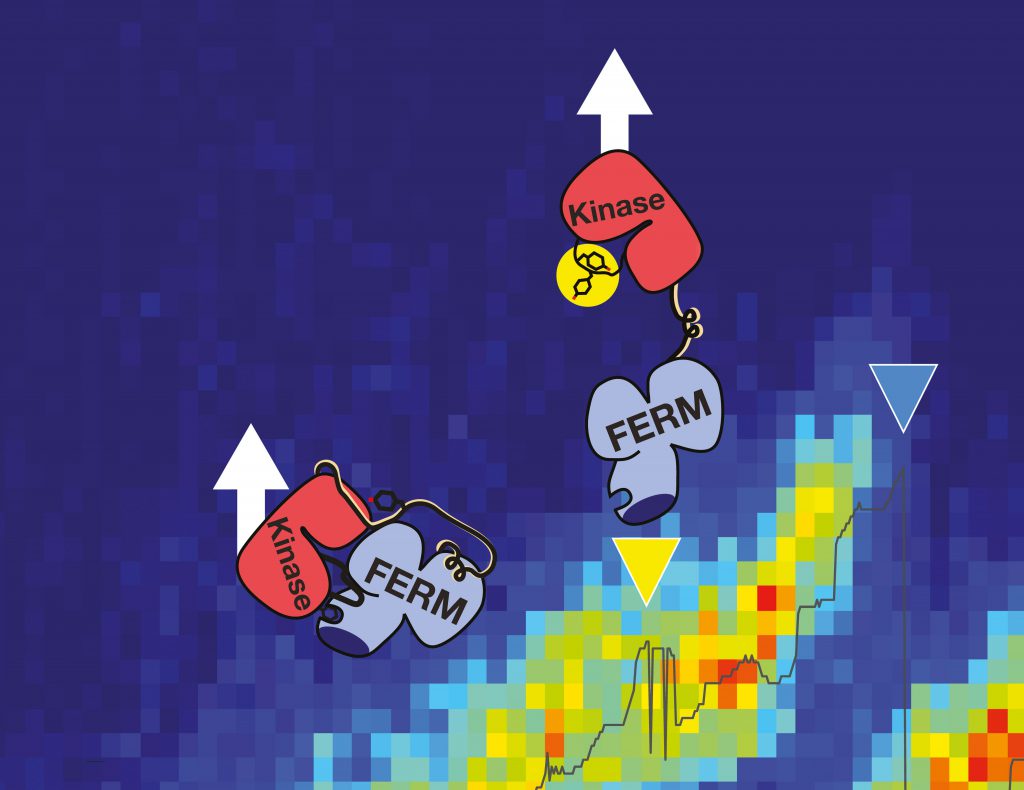
Force for activation
Tumor invasion and metastasis are major problems in the therapy of cancer patients. A team of researchers at the Heidelberg Institute of Theoretical Studies (HITS), the LMU Munich (both Germany) and the CNIO Madrid, Spain, has now elucidated the activation conditions for a key enzyme in this process – a step forward in the development of new therapeutic strategies.
To develop strategies of active tumor metastasis prevention, clarification of the underlying activation process is necessary. Focal adhesion kinase (FAK) is one key enzyme involved in the signaling cascade of cancer invasion and metastasis. This makes it a promising target for cancer therapeutics. NIM scientists in the lab of Professor Hermann Gaub and collaboration partners at the Spanish National Cancer Research Centre in Madrid around Professor Daniel Lietha and at the Heidelberg Institute for Theoretical Studies around Professor Frauke Gräter now elucidated the force-mediated activation process of FAK and present their results in the journal Proceedings of the National Academy of Sciences of the Unites States of America.
What’s the trigger?
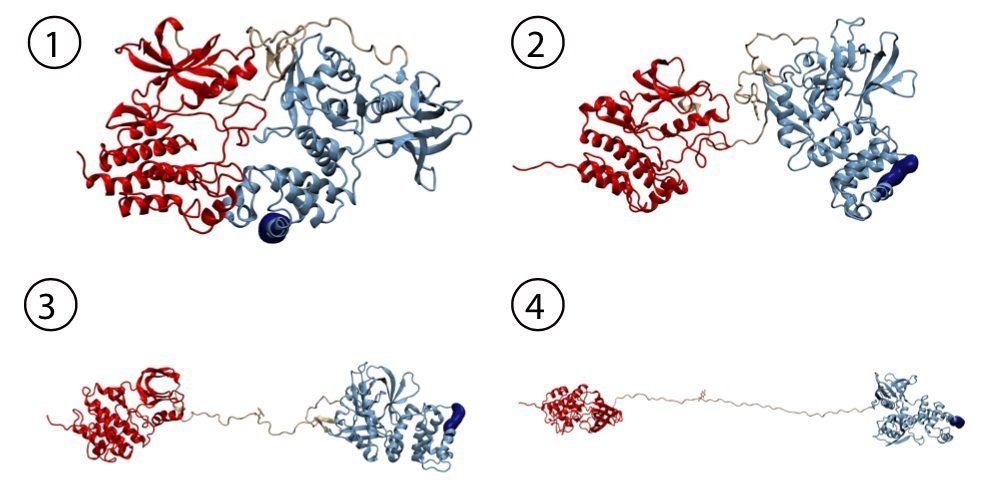
As signaling protein, FAK regulates the essential processes of a cell: adhesion, migration and survival. These kinase proteins are localized at the cytoplasmic site of focal adhesion complexes, figuratively the feet of the cells. “In the non-active state, the focal adhesion kinase is auto-inhibited and inactive with the active center hidden by a protein lid,” explains Magnus Bauer, first author of the publication and NIM-GP graduate student at the LMU. “Our aim was to examine whether mechanical force can open that lid and thereby trigger activation of this key player by inducing conformational changes.” This is of special interest as in pathological situations, activated FAK also promotes adhesion to tumor tissue and the extracellular matrix – required for tumor cell invasion and spreading of metastases. In addition, its overexpression in cancer cells results in the inhibition of natural cell death and downregulation of the tumor suppressor gene p53.
Strong attachment
The mechano-environment of a cell influences its behavior. The focal adhesion kinase acts as sensor for changes in the extracellular und intracellular framework.
“In single-molecule atomic force microscopy experiments and steered molecular dynamics simulations we could show a force-mediated opening of the auto-inhibitory complex of FAK and consequently the activation of the kinase,” describes the physicist Bauer the experimental approach in Munich. The Molecular Biomechanics group at HITS has simulated this process on high performance computers to obtain a fully dynamic view of these events. „We successfully uncovered key steps in FAK activation in great detail,” says group leader Frauke Gräter. “We could show that this signaling protein not only gets activated by force but also stays active while being further stretched out.” In cells, the mechano-activation of FAK is triggered upon stress between the cytoskeleton and the extracellular matrix. “With FAK it seems we found the first non-muscle enzyme to be directly activated by mechanical force,” highlights Bauer. “Transferring our data into cellular systems, we assume that translation of physiological forces into the biochemical signals could be one way to trigger the migration of cancer cells.”
This fundamental insight into the mechanisms of this key enzyme for cancer development opens new routes for future tumor therapy strategies.
Publication:
Structural and mechanistic insights into mechanoactivation of Focal Adhesion Kinase. Bauer MS, Baumann F, Daday C, Redondo P, Durner E, Jobst MA, Milles LF, Mercadante D, Pippig DA, Gaub HE, Gräter F, Lietha D. PNAS, 2019, doi 10.1073/pnas.1820567116.
Scientific Contacts:
Prof. Dr. Hermann Gaub
Biophysics and Molecular Materials
Ludwig-Maximilians-Universität
Amalienstraße 54
80799 Munich
Germany
Phone: +49 (0)89 – 2180 3172
E-Mail: gaub@physik.uni-muenchen.de
Daniel Lietha, PhD
Cell Signaling and Adhesion Group
Structural and Chemical Biology
Biological Research Center (CIB-CSIC)
Calle Ramiro de Maeztu 9
28040 Madrid
Spain
Phone: +34 (0)91 837 3112
E-Mail: daniel.lietha@cib.csic.es
Prof. Dr. Frauke Gräter
Molecular Biomechanics
Heidelberg Institute for Theoretical Studies (HITS)
Interdisciplinary Center for Scientific Computing (IWR), Heidelberg University
Schloss-Wolfsbrunnenweg 35
69118 Heidelberg
Germany
Phone: +49 6221 – 533 267
E-Mail: frauke.graeter@h-its.org
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.