“Something in the way they move” – zooming in on cell migration
Getting from A to B can be a tricky business, especially for cells on the move. An international team of researchers from the Heidelberg Institute for Theoretical Studies (HITS), and the University of Helsinki have zoomed in on the biochemical and biomechanical processes underlying cell migration by taking a closer look at one of the major players, the pseudokinase ILK. The results of their study, published in the Proceedings of the National Academy of Sciences (PNAS), improve our understanding of this intriguing protein.
While the vast majority of cells stay roughly where they are during their lifetime and only travel short distances, there are a few specialized cells which need to move freely and quickly to fulfill their tasks, such as immune cells or the so-called fibroblasts the researchers used in this case, which for example move during wound healing. But whether fast or slow, inert or agile, they need to find the right balance between structural stability and flexibility by sensing and responding to a broad variety of biochemical and mechanical signals from their neighboring cells and the matrix to which they adhere.
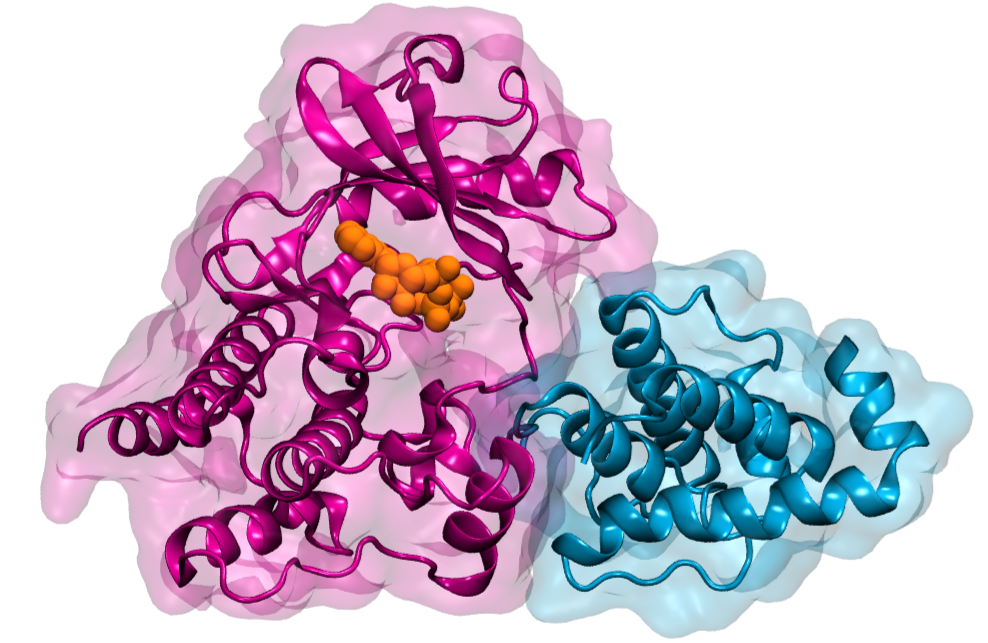
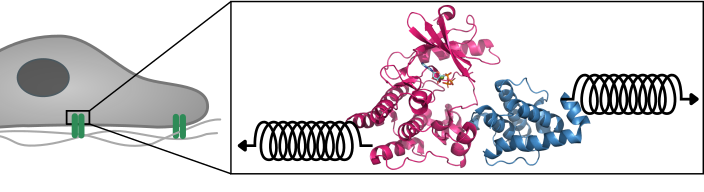
Large protein complexes mediate this signal transduction between the cell and the matrix. One crucial protein in this complex is integrin-linked kinase (ILK). “As a pseudokinase, ILK is not capable to catalyze a chemical reaction, as conventional kinases do,” says first author Isabel Martin from the “Molecular Biomechanics” group (MBM) at HITS. “It was therefore interesting to us why ILK still binds ATP, the small molecule normally used for catalysis, and how that relates to cell motion.”
By combining molecular dynamics simulations with cell biology involving traction force microscopy – the latter two carried out by Sara Wickström’s lab at Helsinki University – Martin and her colleagues investigated the role of ATP-binding to human ILK, and examined the altered kinase dynamics and cell behavior as a result of ATP removal.
“Only by using simulations, we could analyze ILK in molecular detail, and were able to see that ATP gives structural stability to ILK. This effect is the result of an internal force propagation pathway from ATP to residues that bind an important adaptor protein,” Martin says. “Our idea now is that ATP in ILK adopted a new and unforeseen function, namely, to aid ILK to relay mechanical forces by giving it structural stability.”
In a further step they verified the predictions from the simulations and moved beyond their time- and length scales to study the large-scale cellular effects of retained ATP-binding to ILK, for which they joined forces with colleagues in Finland.
“Motivated by the new insights from the computer simulations done at HITS, we tested the predictions by generating cells that contained a mutated ILK protein that is not able to bind ATP. Indeed these cells moved less efficiently than the normal cells where ATP was able to bind and strengthen ILK”, says Sara Wickström, Professor of Cell and Developmental Biology
Faculty of Medicine and Helsinki Institute of Life Science, who led the research at the University of Helsinki.
The surprise was that ATP here does not carry its conventional biochemical role, but is a mechanical stabilizer – a small molecule making a big difference. And Frauke Graeter, head of the MBM group at HITS and co-author of the study, summarizes: “Our findings add another piece in the puzzle to improve our understanding of how cells can stay where they are supposed to but move when they are required to.”
Publication:
Isabel M. Martin, Michele M. Nava, Sara A. Wickström, and Frauke Gräter: ATP allosterically stabilizes integrin-linked kinase for efficient force generation. PNAS, Vol. 119 | No. 11. https://www.pnas.org/doi/10.1073/pnas.2106098119
Scientific contact:
Prof. Dr. Frauke Gräter
Molecular Biomechanics Group (MBM)
Heidelberg Institute for Theoretical Studies (HITS), Germany
https://www.h-its.org/people/prof-dr-frauke-grater/
Isabel Martin
Molecular Biomechanics Group (MBM)
Heidelberg Institute for Theoretical Studies (HITS), Germany
https://www.h-its.org/people/isabel-martin/
Prof. Dr. Sara Wickström
Helsinki Institute of Life Science, Biomedicum Helsinki
University of Helsinki, Finland
Press contact:
Dr. Peter Saueressig
Head of Communications
Heidelberg Institute for Theoretical Studies (HITS), Germany
peter.saueressig@h-its.org
Anu Koivusipilä
Communications specialist
University of Helsinki. Finland
anu.koivusipila@helsinki.fi
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.