Holding on to letting go – the Power of Bonds in Cell Stability
Two-component adhesives are a brilliant invention. Scientists at the Heidelberg Institute for Theoretical Studies (HITS) have found that the principle behind them may also apply to the way certain proteins provide stability within cells – by reinforcing each other to achieve optimal results. Together with scientists in Rehovot, London and Zurich they took a closer look at vinculin and talin, two proteins whose intricate interplay has a pivotal role in forming large and complex cytoskeletal structures. The work was published in Nature Communications.
Many cells in our body respond to a variety of mechanical cues from their immediate surroundings. A major role in this process, known as mechanosensing, is played by so-called focal adhesions, complex structures which serve as contact points for the cell with the extracellular matrix and regulate its communication with the surrounding environment.
One of the key components in focal adhesion development is the so-called talin-vinculin axis, though how the two proteins respond to forces and regulate one another has hitherto remained largely unclear.
“Both components of the glue, talin and vinculin, need force for binding to each other, that is, for becoming sticky”, says Frauke Graeter, who led the research at HITS. “The puzzle we started from was that vinculin needs to stick to talin to actually feel the force in the first place.”
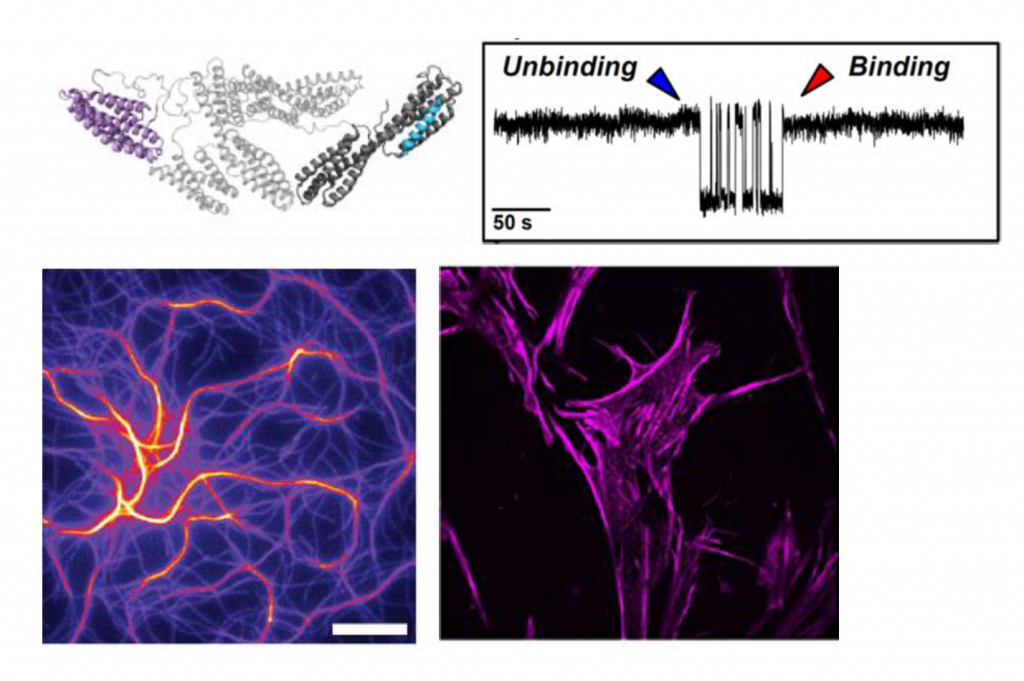
Together with colleagues from the Weizmann Institute, King’s College London and the University of Zurich they tackled this chicken and egg problem of what came first, the force to stick or stickiness to get under force, by combining computational and wet lab techniques. This way, the researchers were able to reveal a two-way allosteric mechanism in full atomistic detail of how vinculin regulates talin binding and vice versa.
“Our answer is that bringing them in touch results in an initially weak binding, but now, having the two components in place and under force, they strengthen each other and become a super glue”, explains Florian Franz, one of the study’s first authors.
The scientists revealed that vinculin matures when talin binds, and in turn stabilizes talin. A critical step was to mimic this mechanism in a vinculin mutant, designed at the computer, which functions in absence of talin or force.
The study also proposes the vinculin mutants as close mimics of vinculin bound to stretched talin, and as useful molecular tools to study and interfere with cell-matrix and cell-cell adhesion, adding another important step toward a better understanding of how cells communicate – and stick.
The study has received funding from the Klaus Tschira Foundation.
Scientific contact:
Prof. Dr. Frauke Gräter
Molecular Biomechanics Group
Heidelberg Institute for Theoretical Studies (HITS)
Press contact:
Dr. Peter Saueressig
Head of Communications
Heidelberg Institute for Theoretical Studies (HITS)
About HITS
HITS, the Heidelberg Institute for Theoretical Studies, was established in 2010 by physicist and SAP co-founder Klaus Tschira (1940-2015) and the Klaus Tschira Foundation as a private, non-profit research institute. HITS conducts basic research in the natural, mathematical, and computer sciences. Major research directions include complex simulations across scales, making sense of data, and enabling science via computational research. Application areas range from molecular biology to astrophysics. An essential characteristic of the Institute is interdisciplinarity, implemented in numerous cross-group and cross-disciplinary projects. The base funding of HITS is provided by the Klaus Tschira Foundation.