New TRAPP webserver for studying TRAnsient Pockets in Proteins
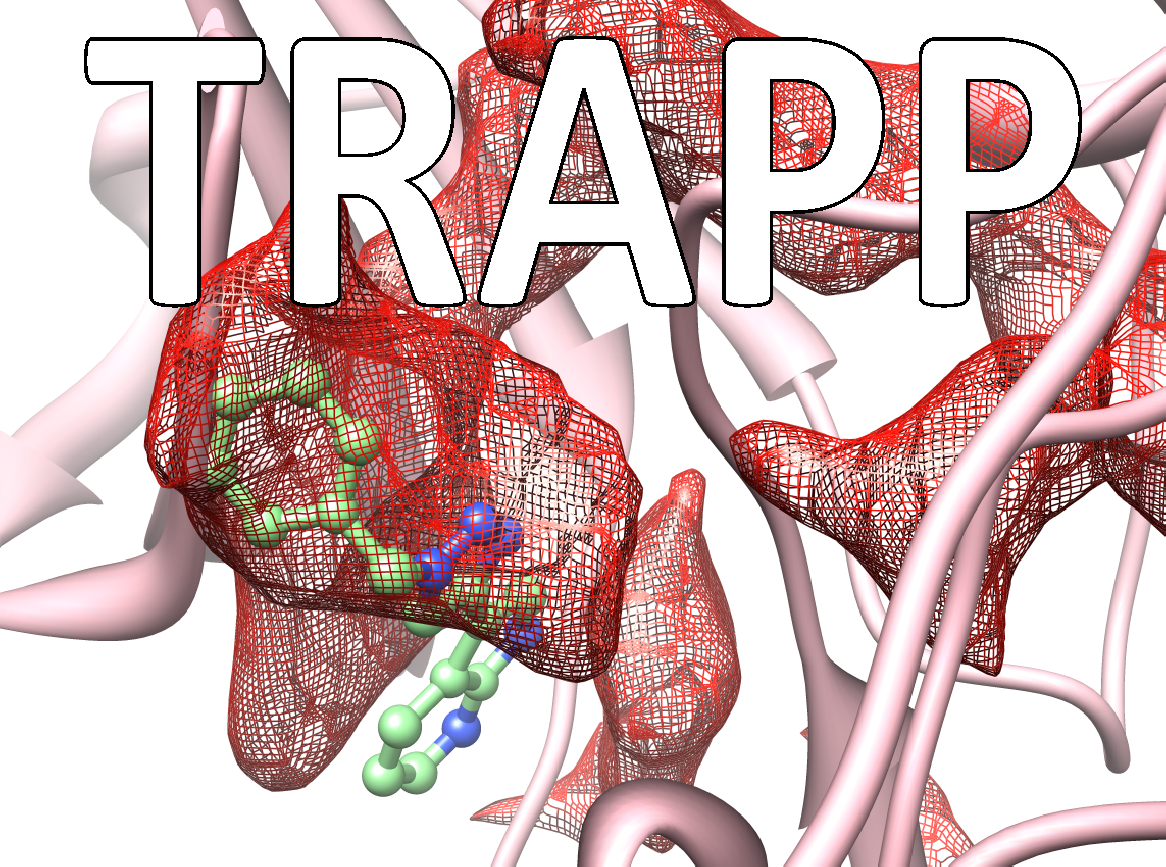
Many drugs exert their therapeutic effects by binding in cavities or pockets on specific target protein macromolecules. The binding pockets on proteins are mobile and dynamic and constantly changing shape. Some pockets or subpockets can be described as transient because they are not always present in the given protein. In structure-based drug design procedures, the dynamic nature of the target protein is usually neglected. A crystal structure of the protein may not reveal transient pockets as it provides a picture of a fixed state of the protein. The analysis of molecular dynamics simulations of a protein can however reveal them. Transient pockets are particularly interesting because they can open and close, allowing ligands to bind. Transient pockets can therefore be exploited to design compounds with greater target specificity or better kinetic binding properties than would be achieved by considering the target as a static structure.
The TRAnsient Pockets in Proteins (TRAPP) webserver, developed in the Molecular and Cellular Modeling (MCM) group at HITS and recently published in the journal Nucleic Acids Research, allows users to employ a range of computational methods to generate snapshots of protein structures, to evaluate them for flexibility, and to detect and characterize transient pockets. The information TRAPP provides can be used to explore protein motion and flexibility, the appearance of transient pockets and the physiochemical, sequence and functional characteristics of binding pockets. The TRAPP webserver provides a user-friendly environment for a dynamic-structure-based approach to drug discovery.
Publication:
Antonia Stank, Daria B. Kokh, Max Horn, Elena Sizikova, Rebecca Neil, Joanna Panecka, Stefan Richter and Rebecca C. Wade. TRAPP webserver: predicting protein binding site flexibility and detecting transient binding pockets. Nucleic Acids Res (2017) gkx277. (Fulltext)
Über das HITS
Das HITS (Heidelberger Institut für Theoretische Studien) wurde 2010 von dem Physiker und SAP-Mitbegründer Klaus Tschira (1940-2015) und der Klaus Tschira Stiftung als privates, gemeinnütziges Forschungsinstitut gegründet. Es betreibt Grundlagenforschung in den Naturwissenschaften, der Mathematik und der Informatik. Zu den Hauptforschungsrichtungen zählen komplexe Simulationen auf verschiedenen Skalen, Datenwissenschaft und -analyse sowie die Entwicklung rechnergestützter Tools für die Forschung. Die Anwendungsfelder reichen von der Molekularbiologie bis zur Astrophysik. Ein wesentliches Merkmal des Instituts ist die Interdisziplinarität, die in zahlreichen gruppen- und disziplinübergreifenden Projekten umgesetzt wird. Die Grundfinanzierung des HITS wird von der Klaus Tschira Stiftung bereitgestellt.