Intrinsically disordered Proteins
Intrinsically disordered proteins (IDPs) are a new class of proteins that lack secondary and tertiary structures and instead explore a broad conformational ensemble. Their functions, from transcriptional regulation to signal transmission, are rather critical and tightly regulated. To quantitively describe the characteristics of IDP’s conformations is thus of great significance.
However, due to high ratio of charged residues or low ratio of hydrophobic residues, computationally derived ensembles from MD simulations show overcompacted conformers compared to experiment. To this end, we have redefined nonbonded interactions, by either increasing water dispersion forces or adopting the Kirkwood-Buff force field to rescue the IDPs from collapsed conformations [1][2].
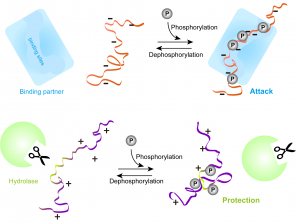
Furthermore, IDPs are subject of extensive reversible post-translational modifications (PTMs), such as phosphorylation, methylation and glycosylation. Among these PTMs, phosphorylation is one of the most common and important PTMs. However, the mechanism of how phosphorylation affects the conformations and functions of IDPs remains unclear. To answer this question, we have performed extensive all-atom molecular dynamics simulations for four representative IDPs: Ash1, E-Cadherin, CTD2’ and p130Cas in their unphosphorylated and phosphorylated forms. Our results showed that all IDPs undergo a mild change upon multi-site phosphorylation, which is V-shaped: phosphorylation moderately expands neutral or overall negatively charged IDPs and shrinks positively charged IDPs. More importantly, in two of these IDPs, only two biologically relevant phosphorylation sites suffice to render the adjacent negatively charged active site significantly more exposed to the environment, which implies a higher probability to interact with other binding partners [3].
1. Mercadante D, Milles S, Fuertes G, Svergun DI, Lemke EA, Gräter F. Kirkwood-Buff Approach Rescues Overcollapse of a Disordered Protein in Canonical Protein Force Fields. J Phys Chem B. 2015.
2. Mercadante D, Wagner JA, Aramburu I V., Lemke EA, Gräter F. Sampling Long- versus Short-Range Interactions Defines the Ability of Force Fields to Reproduce the Dynamics of Intrinsically Disordered Proteins. J Chem Theory Comput. 2017.
3. Jin F, Gräter F. How multisite phosphorylation impacts the conformations of intrinsically disordered proteins. PLoS Comp Biol. In revision.